New Rochelle
NeuroStar TMS
Treatment for Adults & Adolescents
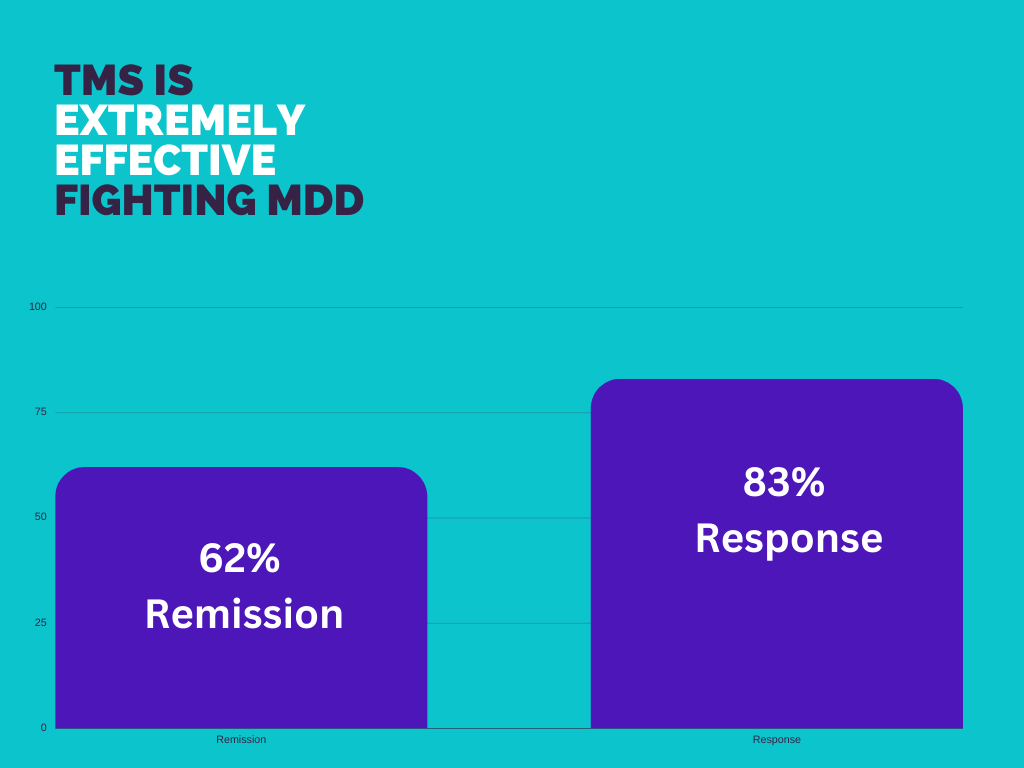
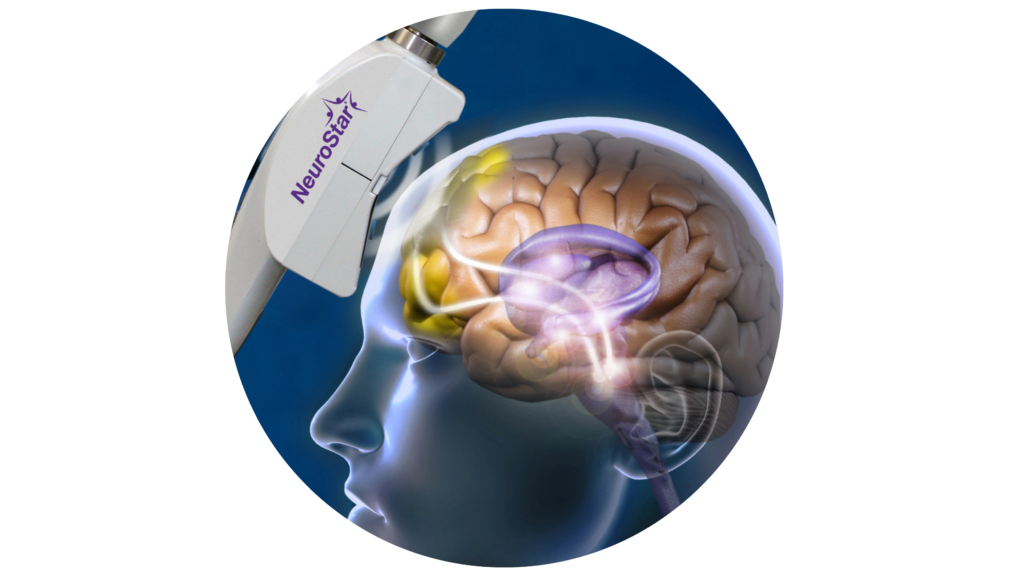
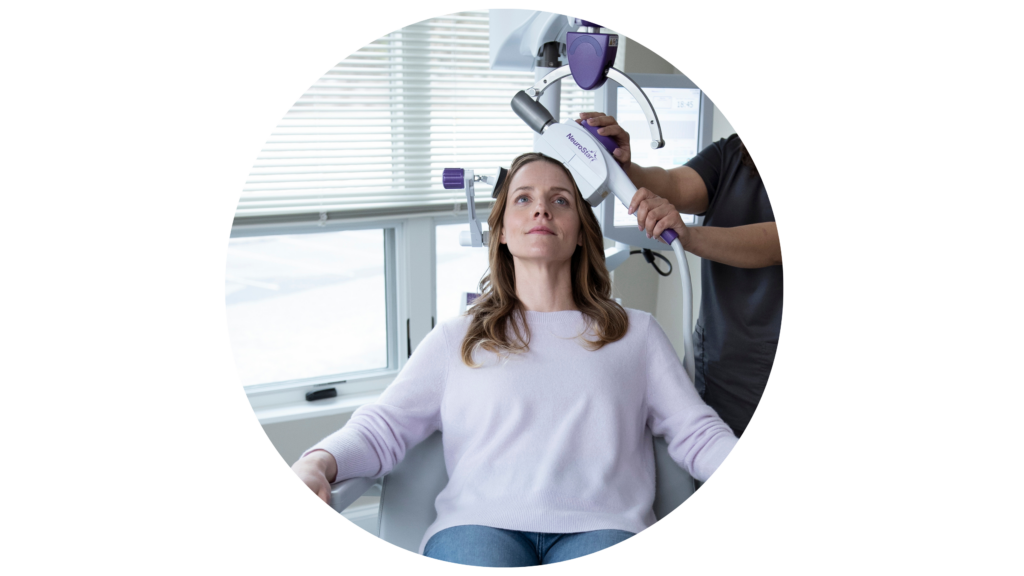
Transcranial Magnetic Stimulation (TMS) is an FDA-approved treatment for severe depression, anxiety, and OCD. It is an amazing treatment option for patients who have tried medication and therapy but haven’t found relief.
For more information, call or text us at (212) 707-8662